
One responder requested price support to minimise the potential for an out-of-stock situation. Supportive of idarucizumab listing, noting the lack of alternatives and likely health benefits.
All responses were supportive of the proposal: Theme All consultation responses received by 8 July 2016 were considered in their entirety in making a decision on the proposed changes. We appreciate all of the feedback that we received and acknowledge the time people took to respond. Dabigatran will remain listed in Section B and Part II of Section H of the Pharmaceutical Schedule without restriction, except that the current restriction in Section B on the 75 mg capsule, limiting use to a maximum of two capsules per day, will continue to apply.Pradaxa will have subsidy and delisting protection until 30 December 2019.A new confidential rebate structure will apply to Pradaxa, reducing the net price.Idarucizumab will be listed under a new Pharmaceutical Schedule subheading, Anticoagulant Reversal Agents in the Antifibrinolytics, Haemostatics and Local Sclerosants therapeutic subgroup of the Blood and Blood Forming Organs therapeutic group), in Part II of Section H of the Pharmaceutical Schedule from 1 September 2016 as follows (ex-manufacturer, excluding GST):.the price and subsidy for all strengths of dabigatran (Pradaxa) will be reduced.ĭetails of the decision Idarucizumab (Praxbind).idarucizumab (Praxbind) will be listed in Part II of Section H of the Pharmaceutical Schedule and.In summary, the effect of the decision is that from 1 September 2016:
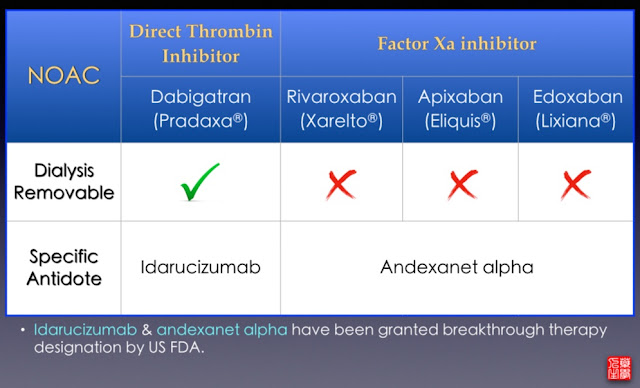
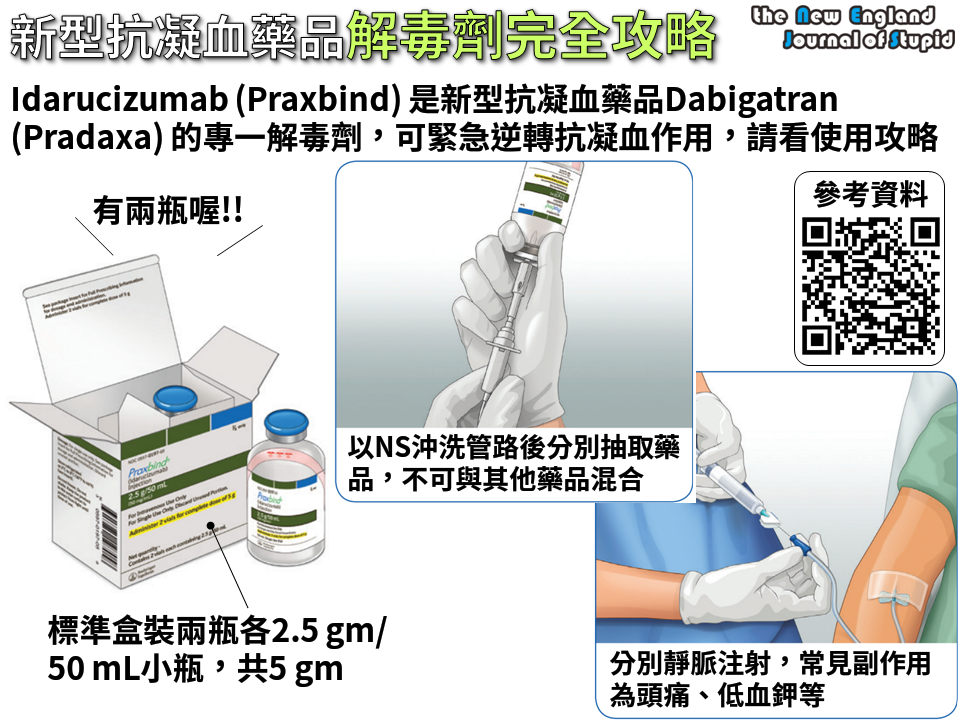
This was the subject of a consultation letter dated 23 June 2016. Idarucizumab can be used in situations where reversal of the anticoagulant effects of dabigatran is required for emergency surgery/urgent procedures, or situations of life-threatening or uncontrolled bleeding. The funding of idarucizumab means that an antidote to dabigatran will be available for use in DHB hospitals from 1 September 2016.


 0 kommentar(er)
0 kommentar(er)
